Information on Angiotensin-I Converting Enzyme (ACE1) Inhibition Activity Testing Service
VISBIO Co., Ltd. offers a service to analyze and test the inhibitory effects of Angiotensin-I Converting Enzyme (ACE1) that affect blood pressure using the Enzymatic Assay method. This service is available for all products in the health and beauty industry that focus on blood pressure regulation.
ACE1 is an essential enzyme in blood vessels that affects blood pressure regulation in patients with high blood pressure, heart disease, and constricted blood vessels. Therefore, studying ACE1 enzyme inhibitors derived from various sources, such as proteins or peptides is important in developing products for patient treatment, herbal products, dietary supplements, or functional food products aimed at reducing blood pressure and enhancing patient care. ACE1 enzyme inhibitors, obtained from various sources, may not be as potent as synthetic inhibitors, but they are free from side effects. Most ACE1 inhibitors are derived from milk products, but peptide inhibitors obtained from animal and marine sources, as well as collagen and gelatin, also have significant inhibitory effects on blood pressure. Therefore, it is important to study and develop these natural ACE1 enzyme inhibitors to contribute to the management and treatment of high blood pressure in patients with heart disease and vascular diseases.
Angiotensin-I Converting Enzyme (ACE1) Inhibition Activity Testing
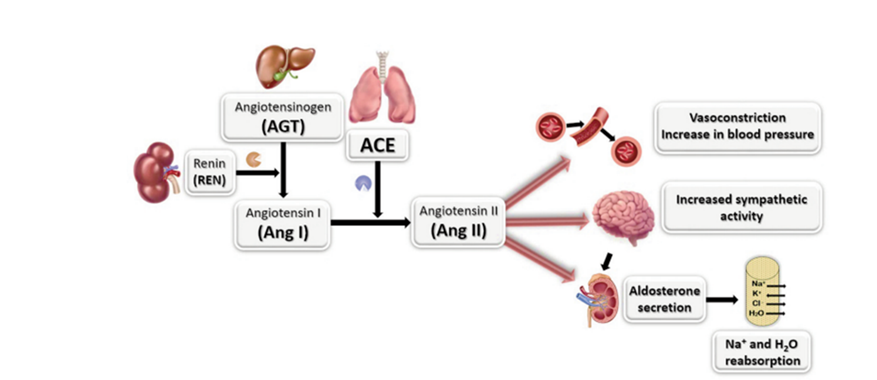
Angiotensin-I Converting Enzyme (ACE1) is a key regulator of blood pressure and plays a crucial role in the renin-angiotensin-aldosterone and kallikrein-kinin systems. ACE enzyme catalyzes the conversion of Angiotensin I to Angiotensin II, leading to vasoconstriction and increased blood pressure. By breaking down dipeptides at the carboxyl terminal, it stimulates various processes in the body, such as contraction of smooth muscle cells in blood vessels, enhancement of sympathetic nervous system activity, and stimulation of the adrenal cortex to release aldosterone, promoting sodium and water retention in the kidneys. Therefore, it is an important part of the renin angiotensin system that controls blood pressure. The study of ACE1 enzyme inhibitors is vital in developing products to manage and treat conditions like high blood pressure, which is a significant target in treating heart and vascular diseases.
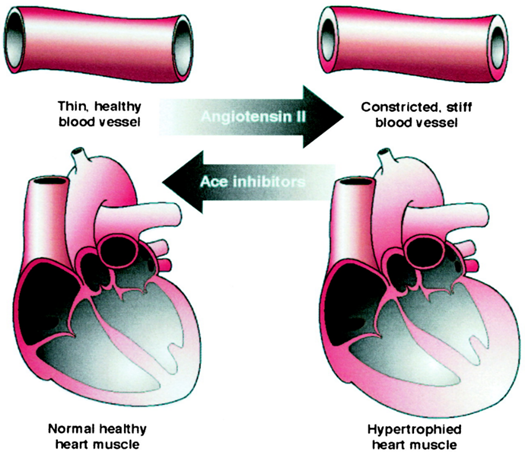
Research has found that ACE1 enzyme promotes the conversion of Angiotensin I to Angiotensin II. When the level of Angiotensin II is high, it thickens and hardens the walls of blood vessels, causing constriction. This may lead to cholesterol accumulation and blockages in the arteries, which can result in coronary artery disease and cerebrovascular diseases. Patients with heart disease and various vascular diseases often have abnormally high levels of Angiotensin II. Thus, ACE1 inhibitors are crucial in preventing the thickening of the heart’s walls and blood vessels and reducing the production of Angiotensin II.
Figure shows the effects of angiotensin II and ACE1 inhibitors on the cardiovascular system.
Sources of raw materials can inhibit Angiotensin-I Converting Enzyme (ACE1) activity.
Raw materials can inhibit Angiotensin-I Converting Enzyme (ACE1) activity, obtained from various sources such as proteins, peptides, and even collagen and gelatin, may not be as potent as synthetic inhibitors. However, peptides have no side effects. The primary sources of peptides that inhibit the ACE enzyme are dairy products. Studies have also extracted peptides from various types of meat, including plant proteins. In addition, collagen and gelatin are good sources of inhibitors for high blood pressure. However, there have been limited studies on digesting collagen and gelatin to produce peptides with these effects. Collagen sources include pig skin, cow skin, chicken skin, and bones. Collagen from marine animals includes fish skin, fish cartilage, fish scales, squid, and sea cucumbers.
Angiotensin-I Converting Enzyme (ACE1) Inhibition Activity Testing by Fluorescence method
The testing procedure is as follows: ACE1 enzyme is mixed with the sample to be tested and allowed to incubate at 37°C for 15 minutes. After incubation, ACE1 Substrate is added, and fluorescence is measured at an excitation/emission wavelength of 340/430 at 37°C for 30 minutes using a Microplate reader. The results are then used to calculate the percentage of ACE1 enzyme inhibitory activity.
Reporting of Test Results
Test results can be reported in two ways:
- Testing the efficacy of the inhibitory effects of the ACE1 enzyme: This test determines if the sample product is effective in inhibiting the ACE1 enzyme’s activity.
- Reporting test results by calculating the half maximal inhibitory concentration (IC50): This reporting method is suitable for raw material samples, herbal products, or extracts that require information on the percentage of inhibition for further product development.
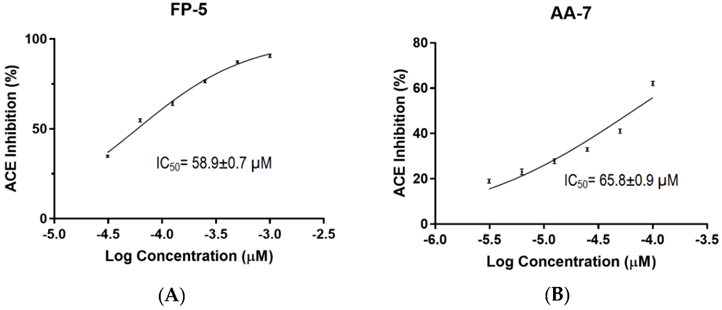
Example of Test Reporting on ACE1 Inhibitory Effects
Peptides derived from Caulerpa lentillifera or Sea Grapes, known as FDGIP (FP-5) and AIDPVRA (AA-7), have IC50 values for ACE inhibition at 58.89 ± 0.68 µM and 65.76 ± 0.92 µM, respectively.
Literature:
- Riordan, J. F. (2003). Angiotensin-I-converting enzyme and its relatives. Genome biology, 4(8), 1-5.
- Masuyer, G., Schwager, S. L., Sturrock, E. D., Isaac, R. E., & Acharya, K. R. (2012). Molecular recognition and regulation of human angiotensin-I converting enzyme (ACE) activity by natural inhibitory peptides. Scientific reports, 2(1), 1-10.
- Gintoni, I., Adamopoulou, M., & Yapijakis, C. (2021). The angiotensin-converting enzyme insertion/deletion polymorphism as a common risk factor for major pregnancy complications. in vivo, 35(1), 95-103.
- Sweitzer, N. K. (2003). What is an angiotensin converting enzyme inhibitor?. Circulation, 108(3), e16-e18.
- Kritsada Pumpeth, Anuchita Moongngarm, and Aswin Amornsin. (2017). Angiotensin I-converting enzyme inhibitory activity of casein hydrolysate, KHON KAEN AGR. J. 45 SUPPL, 1-643
- สามารถ สายอุต, 2561, การผลิตเจลาตินไฮโดรไลเสตที่มีฤทธิ์ยับยั้งเอนไซม์ ACE และต้านอนุมูลอิสระโดยใช้เอนไซม์ papain และเอนไซม์ bromelain, มหาวิทยาลัยบูรพา.