Information on the inhibition efficacy of Phosphodiesterase type 5 enzyme testing service.
VISBIO Co., Ltd. provides testing services for the inhibitory effect of Phosphodiesterase type 5 (PDE5) using Enzymatic Assay for various products, including herbs, extracts, pharmaceuticals, nutritional supplements, and medical devices.
PDE5 is one of the critical Phosphodiesterases (PDEs) that plays a specific role in the cGMP signaling pathway. The cGMP pathway regulates physiological processes such as energy metabolism, intestinal fluid secretion, fat breakdown, egg maturation, cell growth, and anti-inflammatory effects.
Sexual dysfunction is a significant health issue for Thai men, with a prevalence of 37.5%, increasing with age and cardiovascular diseases. Phosphodiesterase-5 inhibitors, the main drugs for treating erectile dysfunction, work by inhibiting the action of the enzyme PDE-5. This inhibition leads to the dilation of blood vessels through the induction of nitric oxide, resulting in penile engorgement.
Enzymes Phosphodiesterase or Phosphodiesterase Type 5.
PDE5 (Phosphodiesterase type 5) is one of the important Phosphodiesterases (PDEs) studied for its mechanism of action. It has a specific target, the cGMP pathway. The cGMP signaling pathway regulates various physiological processes, such as energy metabolism, kidney function, intestinal fluid secretion, intestinal motility, fat breakdown, egg cell maturation, cell growth, and anti-inflammatory responses. Therefore, the function of PDE5 is expressed in various tissues.
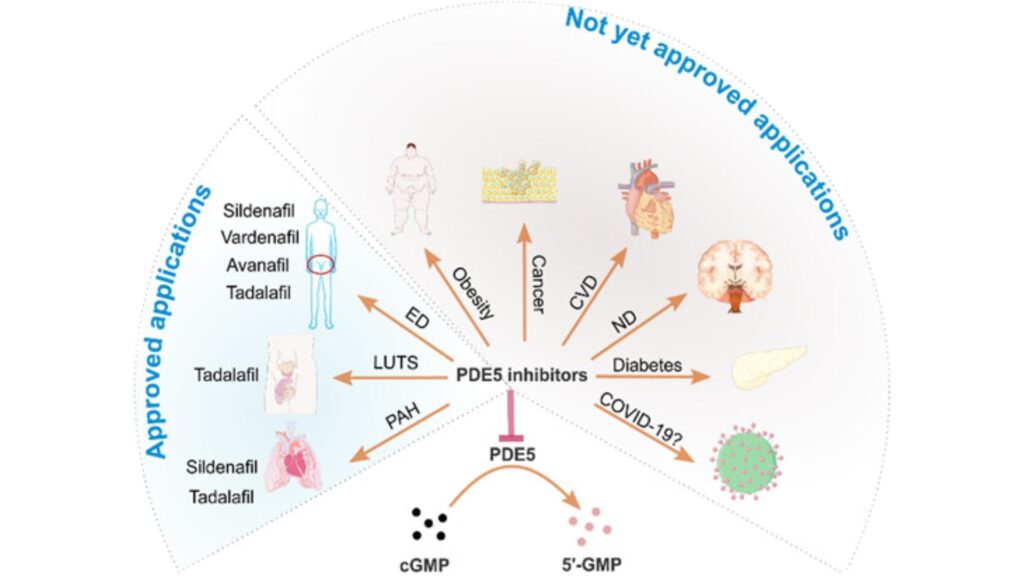
Currently, the pharmacological inhibition of PDE5 (PDE5 inhibitors) has been proven to be beneficial in treating various conditions. These include pulmonary arterial hypertension, lower urinary tract symptoms, cardiovascular diseases, and neurological disorders. Medications in the Phosphodiesterase type 5 (PDE5) inhibitors group, such as Avanafil, Sildenafil citrate, Vardenafil hydrochloride, and Tadalafil, have been certified by the U.S. FDA for the treatment of conditions like erectile dysfunction and pulmonary arterial hypertension.
Phosphodiesterase type 5 inhibitors (PDE5 inhibitors) affect health.
Sexual dysfunction is a significant male health problem as it impacts the quality of life and relationships. In the year 2542 B.E., the prevalence of sexual dysfunction in Thai men was 37.5%, with an increasing trend with age and the presence of heart and vascular diseases. Phosphodiesterase-5 inhibitors are the primary drugs for treating sexual dysfunction. They work by inhibiting the action of the enzyme PDE-5, which breaks down cGMP. When cGMP is not broken down, it leads to the dilation of blood vessels due to the release of nitric oxide and the firmness of the penis.
Risk factors for this condition include heart and vascular diseases, diabetes, high cholesterol, obesity, and smoking. Additionally, medications used to treat high blood pressure or heart diseases may have side effects contributing to erectile dysfunction. Currently, there are various treatment methods available, and many are showing interest in the use of natural extracts for health care. Herbal remedies, especially those with inhibitory effects on the enzyme phosphodiesterase-5 (PDE5 inhibitors), such as Garcinia dulcis or Kaempferia parviflora, have gained significant attention.
Group of medications that exhibit inhibitory effects on Phosphodiesterase type 5 enzyme (PDE5 inhibitors).
Currently, the Thai Food and Drug Administration (FDA) permits the registration of three types of medications for treating erectile dysfunction. These medications possess the inhibitory effect on the Phosphodiesterase type 5 enzyme (PDE5), and they are:
- Sildenafil – Commercially known as Viagra, Elonza, Tonafil, and Sidagra.
- Vardenafil – Marketed under the trade name Levitra.
- Tadalafil – Sold under the trade name Cialis.
In addition, studies have found that Sildenafil, Vardenafil, and Avanafil have the effect of lowering blood pressure. Specifically, Systolic Blood Pressure (SBP) decreases by 8-10 mmHg, and Diastolic Blood Pressure (DBP) decreases by 3-6 mmHg. This reduction starts within 1 hour after taking the medication and lasts for approximately 4 hours. Most patients do not exhibit symptoms due to such changes in blood pressure, except for some individuals with pre-existing low blood pressure or those taking multiple antihypertensive medications or nitrate drugs. There are precautions for patients with cardiovascular disease (Table 1).
Table 1: Categorization of Patients Based on Cardiovascular Risk Before Treatment with PDE-5 Inhibitors
Note*: Risk factors for cardiovascular disease include age, male gender, hypertension, diabetes, hyperlipidemia, smoking, daily lifestyle behavior, and having a family history of premature angina.
The use of PDE-5 inhibitors, including Sildenafil, Tadalafil, Vardenafil, and Avanafil, is considered a primary treatment. While these drugs in this group have similar efficacy, there are differences in pharmacokinetics and adverse effects. Caution must be exercised, especially in patients with cardiovascular conditions. This group of patients often has risk factors that may lead to interactions between medications, potentially causing severe hypotension that could be life-threatening. Therefore, a thorough risk assessment by a physician is essential before prescribing medications from this group.
Methods for Testing the Inhibitory Effects of Phosphodiesterase Type 5 Enzymes (PDE5 Inhibitors)
Phosphodiesterase-5 (PDE-5) enzymes play a crucial role in the degradation of cyclic nucleotides, including cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). Inhibition of PDE-5 reduces the levels of these cyclic nucleotides in various tissues. This enzyme is particularly relevant to addressing erectile dysfunction issues.
The search for substances capable of inhibiting PDE-5 is significant, and preliminary test data can be valuable for the development of highly effective drugs to address sexual dysfunction problems in the future.
Testing the inhibition of PDE-5 involves Enzymatic Assays, where the fluorescence of the sample is measured at Ex/Em = 485/528 nm. The ability to inhibit the function of PDE5 is then calculated. Reporting the test results can be done in two formats:
- Inhibitory Efficacy Report: This format reports the ability of the sample to inhibit the function of PDE5. The results can be presented as a percentage of the inhibitory effect compared to a standard substance.
- IC50 Report: This format involves calculating the half-maximal inhibitory concentration (IC50), representing the concentration of the substance required to inhibit 50% of PDE5 activity. This report is particularly suitable for herbal extracts and raw materials intended for further development in future products.
Examples of research reports on the effect of inhibiting Phosphodiesterase type 5
The study conducted by Kotera, Jun and colleagues in 2012 explored the inhibitory effects of compounds targeting Phosphodiesterase type 5 (PDE5). The experimental approach involved a PDE assay, where PDE enzyme was prepared at 100 micro-liters in 50 millimolar Tris-HCl pH 8. Subsequently, a solution containing 12.5 millimolar MgCl2, 10 millimolar 2-mercaptoethanol, and 0.825 milligrams per milliliter bovine serum albumin was prepared. This mixture was then combined with 200 microliters of the initial cGMP solution and incubated at 37°C for 30 minutes, followed by a 1.5-minute boiling. The reaction was stopped with 500 micro-liters of methanol after adding 100 micro-liters of a 1 mg/ml concentration of Crotalus atrox snake venom, incubating at 37°C for an additional 30 minutes. The results were analyzed using Dowex (1 8, 200 to 400 columns). Notably, Sildenafil citrate and Vardenafil hydrochloride, commonly used for treating erectile dysfunction, demonstrated high inhibitory activity against Phosphodiesterase type 5. Avanafil exhibited the lowest IC50 value at 5.2 nM when compared to other drugs in the study.
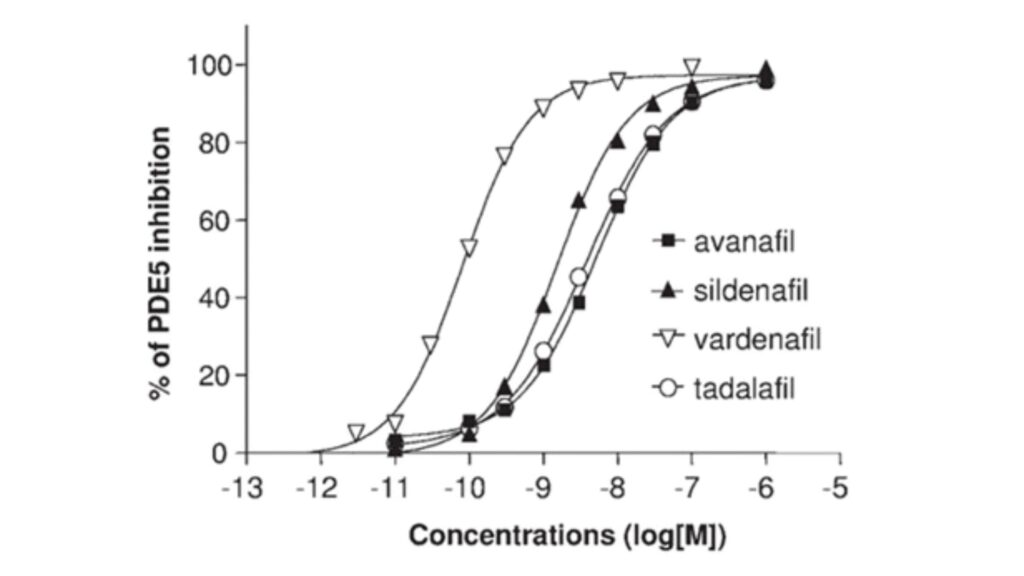
Figure 1: Inhibitory Potency of PDE-5 Inhibitors
Note: The IC50 values represent the concentration of a drug needed to inhibit 50% of the enzymatic activity of PDE-5. A lower IC50 indicates a higher affinity of the drug for the PDE-5 enzyme.
Literature:
- Ahmed, Wesam S., Anupriya M. Geethakumari, and Kabir H. Biswas. “Phosphodiesterase 5 (PDE5): Structure-function regulation and therapeutic applications of inhibitors.” Biomedicine & Pharmacotherapy 134 (2021): 111128.
- Khhumsikiew, Jeerisuda. “Avanafil: ยาใหม่ในกลุ่ม Phosphodiesterase-5 (PDE-5) Inhibitors.” Srinagarind Med J 2014 ;29 (3)
- Kotera, Jun, et al. “Avanafil, a potent and highly selective phosphodiesterase-5 inhibitor for erectile dysfunction.” The Journal of urology 188.2 (2012): 668-674.
- จีริสุดา คำสีเขียว, 2557, Avanafil: ยาใหม่ในกลุ่ม Phosphodiesterase-5 (PDE-5) Inhibitors, ศรีนครินทร์ เวชสาร; 29 (3): 311-320.