Service information on Cellular Toxicity Testing (Toxicity in Cell) for Products
VISBIO Co., Ltd. offers cellular toxicity testing services (Toxicity in cell) in a laboratory setting. Our team of scientific experts conducts tests by cultivating Vero cells in a 2D cell culture. This testing service is applicable to various products in the health and beauty industry, food and beverage industry, herbal industry, pharmaceutical industry and cosmetic industries. The Toxicity in cell testing assesses samples of products such as herbal plants, dietary supplements, herbal products, pharmaceuticals, or cosmetics within the 2D cell culture in the laboratory. This test determines whether the products are toxic to cells and whether they induce cell death. Additionally, the test allows for the calculation of the Cytotoxic Dose (IC50), which represents the concentration at which a product has no adverse effects on cells. The results of these tests can serve as valuable guidance for the development of future products.
A Cytotoxicity Test: What Is It?
A Cytotoxicity Test is a laboratory test conducted to assess the toxicity of various samples to living cells. The test can be applied to a wide range of sample types, including plant extracts, ingredients, raw materials, herbal products, dietary supplements, cosmetics, pharmaceuticals, and more. It involves examining cellular changes, such as cell growth, cell death or other cellular processes. The cells used in cytotoxicity tests can originate from various sources, including animal cells, plant cells or human cells. The test is non-invasive and typically has a short testing duration.
In the testing process, appropriate cells are cultured in a chemical environment containing the substance to be tested. If, after a specified time, the viability of the cells drops below 30%, the sample is considered non-toxic. Conversely, if the cell viability decreases by more than 30%, the sample is considered toxic and does not pass the test.
Cytotoxicity testing serves the purpose of assessing the preliminary safety of a sample and determining the suitable concentration to prevent adverse effects on the body, ensuring safety. Even small quantities of harmful or toxic substances can pose health risks and safety concerns when used.
The Meaning of Vero Cell
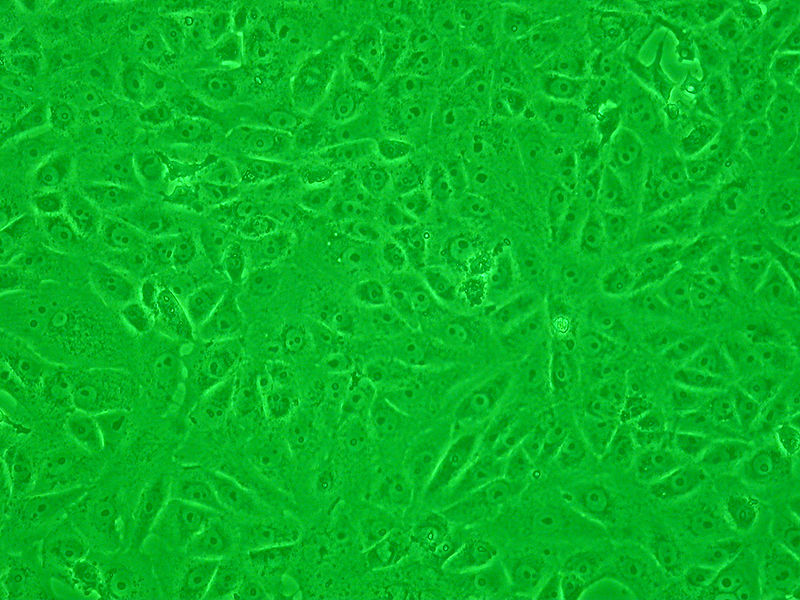
Vero cells are a type of cell commonly used for cell culture and virology research. These cells are derived from the kidney of the African green monkey (Chlorocebus sabaeus). Vero cells are a popular choice among researchers for testing due to their ease of maintenance and high reproducibility.
Vero cells are anchorage-dependent cells, meaning that they grow attached to the surface of the culture vessel. They are generally used for vaccine production and various biotechnological applications, including the isolation and propagation of viruses. Vero cells are utilized in the cultivation of various viruses, such as the poliovirus, rabies virus, and many others. Furthermore, they are employed in toxicology studies where Vero cells are exposed to different samples to assess their cytotoxic effects on living cells.
Cytotoxicity Test Example
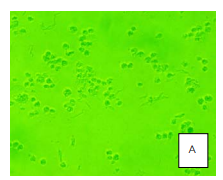
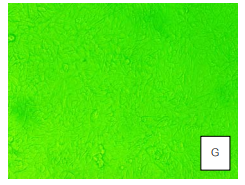
The cytotoxicity test was conducted using herbal extract at concentrations ranging from 2,000 to 31.25 µg/ml. The test was performed on Vero cells at a density of 3 x 105cells/ml in a 96-well plate. The cells were cultured in a CO2 incubator with 5% CO2 at 37 degrees Celsius. The experiment was conducted in four repetitions, and changes in Vero cell conditions were observed daily for a duration of five days. The cytotoxic dose (CD50) was calculated after the test (Chutinan, 2535).
The herbal extract, obtained using ethly alchol, was not soluble in the cell culture medium, so DMSO was used to dissolve it. From a previous study (Chutinan, 2534), it was found that DMSO concentrations of 5% or higher were toxic to Vero cells. Therefore, in the experiment, DMSO at 20% was used to dissolve the herbal extract at a concentration of 1 µg/ml, which was then diluted with water to achieve a concentration of 10,000 µg/ml. The concentration of the herbal extract used for cytotoxicity testing ranged from 2,000 to 31.25 µg/ml. The concentration of DMSO used was less than 0.2%, which was non-toxic to the cultured cells. Under transmission microscopy, the ethly alchol extract of the herbal sample at 2,000 μg/ml (A) was compared to the control image (G). The concentration that resulted in 50% Vero cell death was greater than 2,000 µg/ml (CD50 > 2,000 µg/ml), indicating no cytotoxicity to Vero cells.
Image from: Wannee Chewprecha and Mali Srisodsuk, ‘Toxicity of Thai Herbal Extracts to Mammary Epithelial Cells.’
Literature:
- กลุ่มงานช่วยวิชาการ,เรื่อง การเพาะเลี้ยงเซลล์สัตว์, มหาวิทยาลัยแม่โจ้ : https://erp.mju.ac.th/acticleDetail.aspx?qid=576
- ชุตินันท์ กันตสุข. 2535. การทดสอบเบื้องต้นเพื่อหาฤทธิ์ยับยั้งไวรัสเฮอปีส์ซิมเพลกซ์ของสารสกัดสมุนไพรไทย
- บางชนิด. วิทยานิพนธ์ปริญญาโท, มหาวิทยาลัยจุฬาลงกรณ์.
- วิไลรัตน์ ฉ่ำสิงห์, อรวรรณ์ บุตรดี, ภัทรา มูลจิตรและ สุธี รัตนภิรมย์,”รายงานการศึกษาเบื้องต้น: การใช้ Vero Cell ในการเพาะแยกเชื้อ Newcastle disease virus (NDV) Preliminary Report: Using Vero Cell Line for Newcastle Disease Virus (NDV) Isolation”, KKU Science Journal Volume 40 Number 4, ว.วิทย. มข. 40(4), 2555:1177-1183.
- วรรณี ชิวปรีชา, และ มาลี ศรีสดสุข, “พิษต่อเซลล์ของสารสกัดจากสมุนไพรไทยต่อเซลล์สัตว์เลี้ยงลูกด้วยนม”