
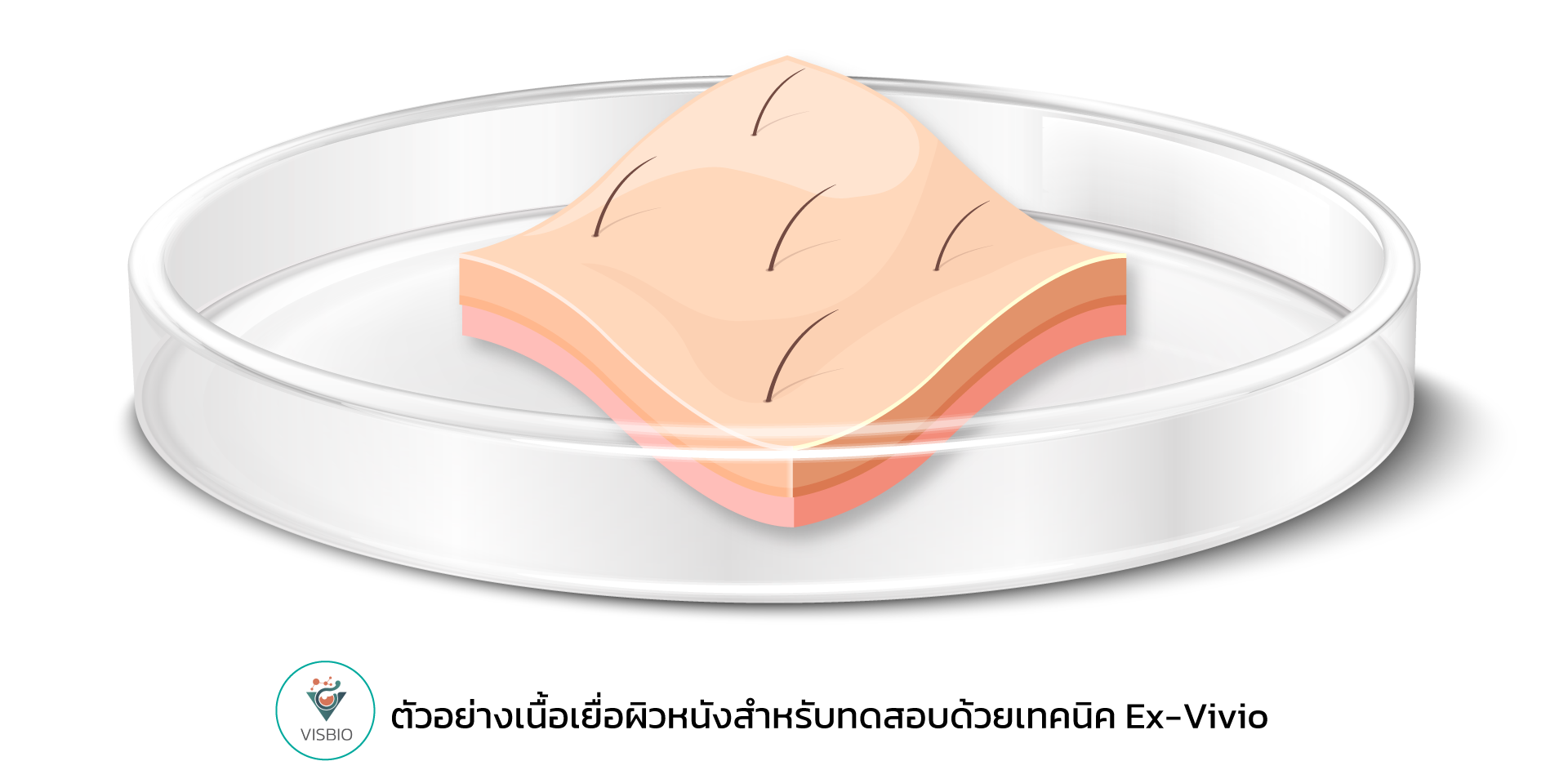
ข้อมูลบริการทดสอบความปลอดภัย Skin Viability ของผลิตภัณฑ์ด้วยเทคนิค Ex-Vivo
Growing concerns regarding product safety and the potential health impacts on consumers worldwide have spurred a demand for testing methodologies that are both reliable and ethically sound. Simultaneously, animal testing and direct human testing are increasingly restricted by stringent ethical limitations, thereby creating an urgent need for testing alternatives that are most physiologically relevant to humans.
Ex-Vivo Human Skin Testing: An Ethically and Scientifically Sound Model
Ex-Vivo Human Skin Testing employs actual human skin tissue obtained through medical procedures, such as cosmetic surgery. These tissues are meticulously maintained under controlled laboratory conditions to closely simulate the physiological state of human skin. A key advantage of this method is its preservation of the complex skin architecture, including the diversity of cell types and metabolic mechanisms, which are notably absent in simpler in-vitro cell cultures and can differ significantly from in-vivo animal models. Consequently, the ex-vivo model functions as a bridge by providing a level of complexity closely approximating human physiology while enabling efficient experimental control.
Key Analytical Tools in Ex-Vivo Testing
1. MTT Assay: Evaluation of Cellular Viability and Response
- The MTT Assay is a standardized technique used to assess cell viability, proliferation, and cytotoxicity. In the context of product safety testing, this method evaluates the impact of test substances on skin cells by monitoring the reduction in viable cells. If a test substance exhibits cytotoxicity, the affected cells will demonstrate a diminished ability to convert the MTT reagent into purple formazan crystals; a decrease in color intensity indicates cytotoxicity. The MTT Assay is both convenient and rapid, yielding quantitative and highly reliable results, making it ideally suited for screening ingredient safety.

2. LDH Assay: Indicator of Skin Cell Damage
- The LDH Assay serves as another crucial tool for assessing cytotoxicity by measuring the quantity of lactate dehydrogenase (LDH) enzyme released from cells with compromised membrane integrity. Under normal conditions, LDH resides within the cell; however, when cells are damaged by toxic substances, LDH is released into the extracellular space. The measured LDH levels directly correlate with the degree of cell damage or death, thus providing a clear indication of skin cell toxicity. The assay is highly sensitive, does not require radioactive substances, and is simple to perform, making it a popular choice for product safety evaluations.
Elevating Reliability: The Rationale for Ex-Vivo Human Skin Testing
The Ex-Vivo Human Skin Model offers a superior physiological framework for product safety assessments by closely replicating the structure and functionality of actual human skin. Its capacity to maintain complex components—such as immune cells—renders it an invaluable tool for accurately investigating the effects of products on human skin. This model is applicable to various tests including irritation testing, sensitization testing, and the evaluation of skin penetration of different substances.
Applications of Ex-Vivo Skin Testing: Encompassing Cosmetics, Pharmaceuticals, and Extracts
Ex-Vivo techniques have emerged as essential instruments in the safety assessment of a wide array of products:

- Cosmetics: Ex-Vivo testing represents a reliable alternative to animal testing for cosmetic safety evaluations, in compliance with international regulations. It can assess the toxicity of both ingredients and finished products, and it facilitates the study of active ingredient penetration to confirm efficacy and safety. This approach underpins consumer assurance in claims of safe cosmetics.

- Pharmaceuticals (Topical Medications): In Vitro Permeation Testing (IVPT) using ex-vivo human skin is widely employed to evaluate the safety of topical drugs. This method enables researchers to assess absorption, retention, and to compare the efficacy of various formulations on real skin under both normal and pathological conditions. Additionally, it provides insights into the mechanisms of action at the gene and protein levels.

- Extracts & Chemicals: The ex-vivo model is beneficial for assessing the safety of natural extracts or chemicals that may come into contact with the skin. It is used to evaluate potential skin irritation, corrosion, and sensitization, as well as to understand the pathways of exposure and rates of skin absorption. This approach is an integral component of non-animal testing strategies that are increasingly accepted.
Ex-Vivo Human Skin Testing for product safety not only represents an ethical alternative but also delivers highly accurate and reliable results due to its close physiological resemblance to human skin. It stands as a critical tool in enhancing safety standards and fostering confidence among manufacturers and consumers alike in today’s marketplace.
Samples of Testable Products and Substances
Ex-Vivo techniques can be employed to assess the safety and properties of various samples of products and substances relevant to the skin, as exemplified below:
1. Products
- Cosmetic Samples: Including skincare product samples, cleanser samples, makeup samples, sunscreen samples, and hair care product samples.
- Topical Medication Samples: Encompassing cream samples, gel samples, ointment samples, and patch samples applied to the skin for treatment purposes.
- Other Personal Care Product Samples: Including baby care product samples, oral care product samples (in terms of lip area irritation), deodorant samples, and hand sanitizer samples.
2. Active Ingredients
- Samples of key substances used in cosmetics for specific effects, such as moisturizers, anti-aging compounds, and whitening agents.
- Samples of active pharmaceutical ingredients used in topical medications for treatment.
- Samples of natural extracts with biological activity.
3. Medicine – Any products for Topical Use
- Samples covering all forms of medication applied externally to the body for treating skin conditions or localized symptoms.
Literature:
- Scott X Atwood and Maksim V Plikus, 2021, Fostering a healthy culture: Biological relevance of in vitro and ex vivo skin models, Exp Dermatol. 2021 Feb 10;30(3):298–303. doi: 10.1111/exd.14296
- Gorkun, A. A., Mahajan, N., Willson, K., Jorgensen, A. M., Wagner, G. A., Kasula, V. R., Jacobson, A., Atala, A., & Soker, S., 2023, Fabrication of Ready-to-Use Ex Vivo Human Skin Models for Chemical Testing: Current Status and Challenges, Skin 3-D Models and Cosmetics Toxicity (pp.19-37)
- Messager, S., Hann, A. C., Goddard, P. A., Dettmar, P. W., & Maillard, J.-Y., 2003, Assessment of skin viability: Is it necessary to use different methodologies. Skin Research and Technology, 9(4):321-30

