Service information for testing the inhibitory effects on cervical cancer cells (Hela)
VISBIO Co., Ltd. provides services for testing and analyzing inhibitory effects on cervical cancer cells (Cervical cancer cells – Hela) in a laboratory setting. Our team of scientific experts conducts tests by culturing cancer cells under 2D cell culture conditions. This testing is suitable for in-depth research aimed at developing herbal products capable of inhibiting cervical cancer cells.
Cervical cancer is the second most common cancer among Thai women, typically affecting those aged 35-60. It is caused by HPV infection. Cervical cancer cells (Hela) are human-derived cervical cancer cells commonly used for the study of cervical cancer biology, particularly in researching the inhibition of these cancer cells outside or within microplates (2D or 3D Cell Culture – Anti-cancer) in cell culture media. Presently, various herbal products, plant extracts, dietary supplements, or medicines are being developed to nourish or combat inflammation in cervical cancer. The product is intended to nourish or combat inflammation of the cervix. Testing its effectiveness at the cellular level can determine its impact, including toxicity to cancer cells, inhibitory effects, or the appropriate concentration needed to inhibit cervical cancer cells (HeLa).
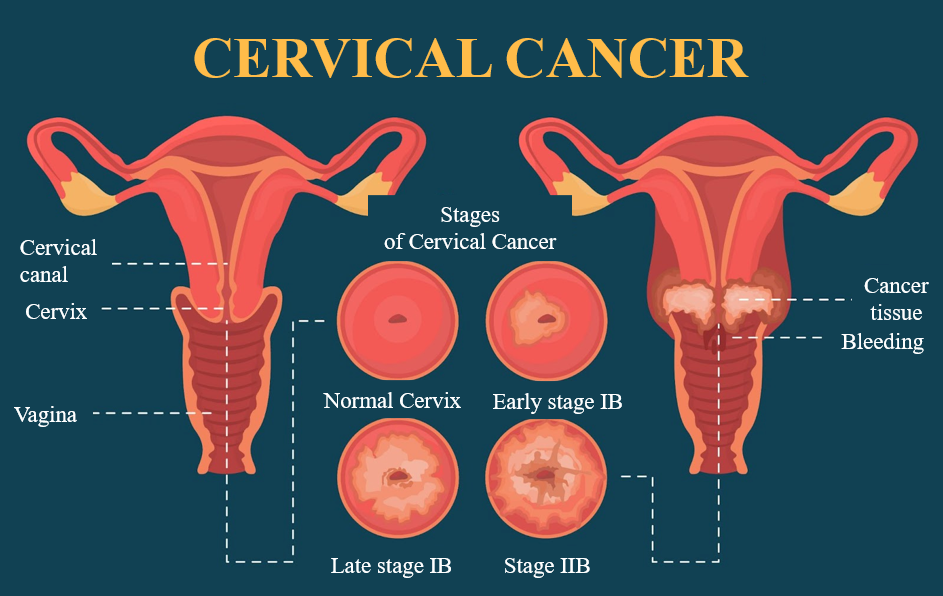
Get to Know Cervical Cancer
Information from the National Cancer Institute in 2021 found that cervical cancer is the second most common cancer among Thai women. It is most prevalent in women aged 35 to 60. The primary cause of cervical cancer is the human papillomavirus (HPV) infection, specifically HPV subtypes 16 and 18, which are the most common in cervical cancer. HPV is typically transmitted through sexual intercourse, putting all sexually active women at risk. Fortunately, about 80-90% of individuals infected with HPV can naturally clear the infection through their immune system. However, in some cases, the body may not be able to clear the infection.
The process of HPV infection can span 5-15 years, causing abnormal changes in cervical tissue, which may eventually progress to cervical cancer. Other risk factors may also contribute, including being over 40 years old, having multiple sexual partners, early sexual activity (before age 18), smoking or exposure to secondhand smoke, having many children, immunodeficiency, and lack of regular screenings.
Cervical cancer is often asymptomatic in its early stages but may present symptoms as the disease progresses. These symptoms can include abnormal vaginal bleeding, pain and bleeding after sexual intercourse, postmenopausal bleeding, irregular, persistent, or heavy vaginal bleeding, bleeding combined with unusual vaginal discharge, and pelvic pain.
Cervical Cancer Cells (Hela)
Cervical cancer cells, known as Hela cells, are human-derived cervical cancer cells commonly used in the field of cervical cancer biology research. They are utilized to study the inhibition of cervical cancer cell growth, both outside the cells and within Well Plates, under two-dimensional or three-dimensional conditions (2D or 3D Cell Culture – Anti-cancer) in cell culture media.
Testing Method for Cervical Cancer Cells (Hela) in Cell Culture - Anti-cancer
Currently, there is development of herbal products, herbal extracts, dietary supplements, or medicines aimed at nourishing or reducing inflammation in cervical. This allows the evaluation of product efficiency at the cellular level, related to cytotoxicity, inhibitory properties, or suitable concentration for inhibiting large intestine cancer cells of the Cervical cancer cells (Hela) type.
The testing process involves taking cells or tissues directly from living organisms and culturing them under conditions where the environment can be controlled. This allows cervical cancer cells to grow, proliferate, or increase in number, closely resembling cancer cells grown in laboratory animals or within a patient’s body. Studying at the cellular level is an initial test before proceeding to analyze further in animal experiments or clinical trials.
Figure demonstrating an example of two-dimensional cell culture in an in-vitro setting used for studying cell biology and cancer research (Gaebler et al., 2017).
Testing the Inhibitory Effect on Cervical Cancer Cells (Hela) Using the MTT Assay
The testing of the inhibitory effect on Cervical cancer cells (Hela), cultivated at the cellular level or in a Well Plate, is conducted using the Methyl tetrazolium 3-[4, 5-Dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) assay. The MTT assay is a method for testing the inhibitory effect on cancer cells in microplates based on the functioning of dehydrogenase enzymes in mitochondria. The yellow MTT (3-[4, 5-Dimethylthiazol-2-yl]-2,5-Diphenyltetrazolium Bromide) dye is converted into a purple formazan precipitate.
Therefore, formazan is used to indicate cell viability. Dead cancer cells will appear colorless, and live cancer cells will produce purple formazan inside the cells. When dissolved in a solvent like DMSO, it results in a blue-purple solution that can be quantified for absorbance using a spectrophotometer. This absorbance is directly proportional to the amount of live cells. Subsequently, the data is used to calculate the percentage of cell survival compared to a standard substance, reporting the results as the percentage of cancer cell inhibition or Half maximal inhibitory concentration (IC50).
Literature:
- มะเร็งปากมดลูกป้องกันได้ด้วยวัคซีน HPV, โรงพยาบาลศิริราช ปิยะมหาราชการุณย์
- มะเร็งปากมดลูก, คณะแพทยศาสตร์ โรงพยาบาลรามาธิบดี มหาวิทยาลัยมหิดล
- ปิยวัฒน์ เลาวหุตานนท์, มะเร็งปากมดลูก, สถาบันมะเร็งแห่งชาติ กรมการแพทย์ กระทรวงสาธารณสุข
- แผนยุทธศาสตร์สถาบันมะเร็งแห่งชาติ,สถาบันมะเร็งแห่งชาติ กรมการแพทย์ กระทรวงสาธารณสุข, พ.ศ. 2562 – 2565
- ทะเบียนมะเร็งระดับโรงพยาบาล, สถาบันมะเร็งแห่งชาติ กรมการแพทย์ กระทรวงสาธารณสุข, พ.ศ.2564
- Gaebler M, Silvestri A, Haybaeck J, Reichardt P, Lowery CD, Stancato LF, et al. Three-dimensional patient-derived In vitro sarcoma models: promising tools for improving clinical tumor management. Front Oncol 2017; 7: 203.