Testing Service for Cholangiocarcinoma Inhibition Activity in Human Cholangiocarcinoma Cell (RBE)
VISBIO Co., Ltd offers testing and analysis services for inhibitory effects on human cholangiocarcinoma cancer cells (RBE) in laboratory settings. Our team of scientific experts conducts cell culture tests in a 2D cell culture. This type of testing is suitable for in-depth research with the goal of developing herbal remedies that can effectively inhibit cancer cells.
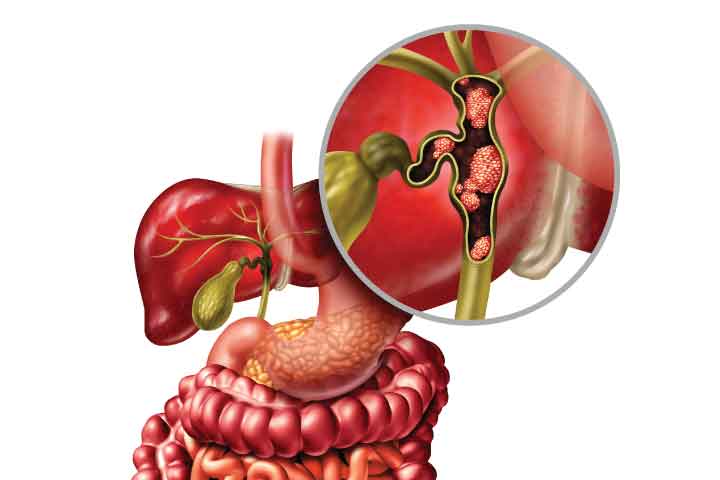
Cholangiocarcinoma is a relatively rare form of cancer worldwide but is more prevalent in Thailand. It ranks among the top causes of cancer-related deaths in the country. Statistics in Thailand indicate that this disease occurs more frequently in males than females, with a higher incidence in the northeastern region, an area known for liver fluke infection.
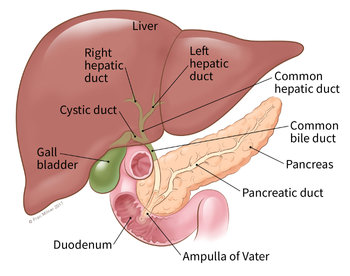
Cholangiocarcinoma can develop in various parts of the bile duct, including within the liver (intrahepatic) and outside the liver (extrahepatic). Currently, there is ongoing development of herbal products, extracts, dietary supplements, or medications aimed at nurturing or preventing inflammation in the bile duct. These products can be tested for their effectiveness at the cellular level, particularly regarding their cytotoxicity towards human cholangiocarcinoma cell lines (RBE).
Getting to Know Bile Duct Cancer or Cholangiocarcinoma
Bile duct cancer, also known as cholangiocarcinoma, is a relatively rare form of cancer worldwide but is more prevalent in Thailand. It ranks among the leading causes of cancer-related deaths in Thailand. Statistical data from Thailand shows that this disease is more common in males than females, with a higher incidence in the northeastern region, an area known for liver fluke infections.
Bile duct cancer can develop in various parts of the bile duct, including within the liver (intrahepatic) and outside the liver (extrahepatic). The symptoms can vary depending on the location of the cancer. Risk factors for developing bile duct cancer include liver fluke infections, particularly in the northeastern region, where the consumption of raw or undercooked fish dishes like “pla ra” (fermented fish) and “koi pla” (raw fish salad) is common. These dishes may contain parasite eggs that can hatch and inhabit the bile duct, leading to chronic inflammation and the potential development of bile duct cancer. Furthermore, bile duct cancer can arise from conditions with abnormal bile duct development, such as choledochal cysts, chronic inflammation of the bile duct, hepatitis B or C infections, cirrhosis, smoking, alcohol consumption, or a family history of bile duct cancer. These factors increase the risk of developing bile duct cancer.
Human Cholangiocarcinoma Cell Lines (RBE) for Bile Duct Cancer Research
Human cholangiocarcinoma cell lines (RBE) are cancer cells originating from humans and are commonly used for the study of bile duct cancer biology. These cell lines belong to a specific type of bile duct cancer cells and are frequently employed to investigate the inhibition of cholangiocarcinoma cells’ growth and proliferation. Researchers study the anticancer properties of substances both outside and within the cells, often in well plates under 2D or 3D cell culture as part of anticancer research.
Testing Human Cholangiocarcinoma Cell Lines (RBE) for Anti-Cancer Cell Culture
Currently, there is a development of herbal products, herbal extracts, dietary supplements, or medications aimed at nourishing or preventing inflammation of the bile duct. These products can be tested for their effectiveness at the cellular level regarding their toxicity to cancer cells, inhibitory properties, or the appropriate concentration required to inhibit Human cholangiocarcinoma cell lines (RBE).
The testing process involves taking cells or tissues directly from living organisms and culturing them under controlled environmental conditions to allow cancer cells to grow, divide, or increase in number. This closely resembles cancer cells grown in laboratory animals or those found in the bodies of patients. Studying at the cellular level is an initial test before proceeding to further analysis through animal experiments or clinical trial testing.
Figure showing an example of 2D in-vitro cell culture of cancer cells used for biological and cancer studies (Gaebler et al., 2017).
Testing the Inhibition Efficacy on Human Cholangiocarcinoma Cell Lines (RBE) Using the MTT Assay
The inhibition efficacy on human cholangiocarcinoma cell lines (RBE) is tested at the cellular level or in Well Plate using the MTT (Methyl tetrazolium 3-[4, 5-Dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay. The MTT assay is a method for testing the inhibitory effects on cancer cells in microplates based on the enzymatic activity of dehydrogenases in mitochondria. The yellow MTT (3-[4, 5-Dimethylthiazol-2-yl]-2,5-Diphenyltetrazolium Bromide) dye is transformed into a purple formazan product.
Thus, the formazan indicates cell viability, where dead cancer cells appear colorless, and live cancer cells exhibit purple formazan within the cells. When dissolved in a solvent like DMSO, it yields a blue-purple solution that can be quantified for absorbance using a spectrophotometer, directly proportional to the amount of live cells. This is then used to calculate the percentage of cell survival compared to a standard substance, reporting the results as % cell inhibition or the concentration of the substance that inhibits cancer cell growth by 50% (IC50).
Literature:
- โรคมะเร็งท่อน้ำดี, มูลนิธิมะเร็งท่อน้ำดีแห่งประเทศไทย
- มะเร็งท่อน้ำดี, โรงพยาบาลจุฬาลงกรณ์ สภากาชาดไทย
- แนวทางการตรวจคัดกรองวินิจฉัยและรักษาโรคมะเร็งตับและท่อน้ำดี, กลุ่มงานสนับสนุนวิชาการ สถาบันมะเร็งแห่งชาติ กรมการแพทย์ กระทรวงสาธารณสุข, 2554
- Gaebler M, Silvestri A, Haybaeck J, Reichardt P, Lowery CD, Stancato LF, et al. Three-dimensional patient-derived In vitro sarcoma models: promising tools for improving clinical tumor management. Front Oncol 2017; 7: 203.