Information on Antioxidant Activity Testing Services for Various Products using DPPH or FRAP or ORAC Methods.
VISBIO offers services for the examination and analysis of antioxidant capabilities in various products. This testing is suitable for products that emphasize the ability to resist free radicals. The underlying principle of free radical formation is a natural mechanism of substances that attract electrons from surrounding molecules, resulting in low stability and susceptibility to reactions. These factors contribute to abnormal cellular functions, causing facial wrinkles and potentially leading to various diseases. Antioxidants are substances that help prevent or inhibit oxidation reactions and come in various types. There are several methods for testing, each with its advantages, disadvantages, and suitability for different substances. The values obtained from each testing method may not correlate directly due to variations in reaction mechanisms during the testing process.
Mechanism of Free Radical Generation
Free radicals are natural mechanisms of substances that attract electrons from other substances in the surrounding area of a molecule or atom. This leads to low stability and high reactivity, resulting in reactions with other molecules in the vicinity. The neighboring molecules then lose or accept electrons, transforming into new free radicals, causing a chain reaction. This chain reaction is a contributing factor to the abnormal functioning of cells, which can result in various conditions such as skin aging and the development of diseases like cancer and cardiovascular diseases.
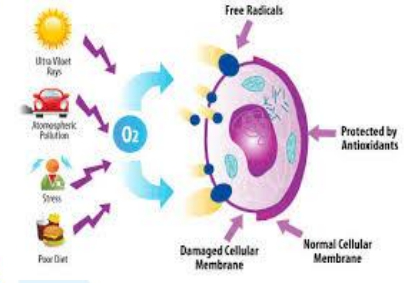
Antioxidant Compounds
Antioxidants are substances that help prevent or inhibit oxidation reactions. They can be classified into five types:
- Primary antioxidants: These are phenolic compounds that act as chain terminators in the lipid peroxidation reaction, providing electrons.
- Oxygen scavengers: Examples include ascorbic acid (vitamin C), which reacts with oxygen.
- Secondary antioxidants: Examples include thiopro-pionic acid, which helps break down lipid hydroperoxide.
- Enzymatic antioxidants: Examples include Superoxide dismutase (SODs) and Catalase (CAT), which eliminate oxygen and free radicals.
- Metal chelating agents: Examples include citric acid and amino acids, which bind to metal ions.
These antioxidant compounds are predominantly found in herbs, vegetables, and fruits, such as phenolic compounds, carotenoids, and flavonoids.
Testing Antioxidant Capacity
There are several methods used to assess the antioxidant capacity of herbs, vegetables, fruits, cosmetics, and dietary supplements. Here are a few examples:
1. DPPH assay: This method utilizes the color change of 2,2-diphenyl-1-picrylhydrazyl (DPPH) when it reacts with substances possessing antioxidant properties. The purple color of the DPPH solution changes to yellow when a sample with high antioxidant capability is present.
2. FRAP assay: FRAP (Ferric Reducing Antioxidant Power) measures the ability of a substance to reduce Fe3+-TPTZ (ferric tripyridyl triazine) to Fe2+-TPTZ (ferrous tripyridyl triazine). This results in a blue color change.
3. ORAC assay: ORAC (Oxygen Radical Absorbance Capacity) measures the ability of a substance to inhibit the formation of peroxyradicals, which stops the chain reaction by passing hydrogen atoms. It involves using βphycoerythrin (fluorescein), which exhibits fluorescent properties. The β-phycoerythrin is destroyed by free radicals, causing a decrease in its fluorescence.
There are several methods to test for antioxidant activity, each of which has its advantages, disadvantages, and suitability for different test substances. The values obtained from each testing method may not be directly comparable because the reaction mechanisms involved in the tests can vary. Additionally, there are various types of test substances found in herbs, vegetables, and fruits that exhibit antioxidant properties, some of which contain radicals and others that do not.
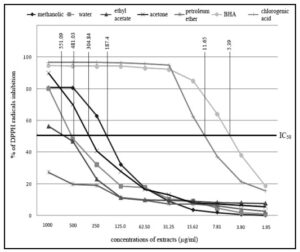
Example of reporting the results of free radical scavenging activity (IC50) using the DPPH assay.
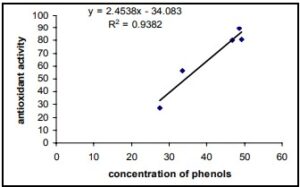
The report presents the results of testing the antioxidant activity using the DPPH assay (Milan, 2011). The analysis of the IC50 values from Figure 1 shows the DPPH values of various plant extracts compared to the standard BHA value. To calculate the IC50 values of different substances, the values should be calculated using the linear equation shown in Figure 2.
Literature:
- Shahidi, F., 1997, Natural Antioxidants : an Overview, in F. Shahidi (Ed.), Natural Antioxidants: Chemistry, Health Effects and Applications, AOCS Press, Illinois, pp. 1-7.
- Milan S. Stankovi, 2011, TOTAL PHENOLIC CONTENT, FLAVONOID CONCENTRATION AND ANTIOXIDANT ACTIVITY OF Marrubium peregrinum L. EXTRACTS, Kragujevac J. Sci. 33 (2011) 63-72.
- Patiwit Loypimai, 2012, Total Antioxidant Capacity Assessments invitro, J Sci Technol MSU, Vol 31, No 2.
- โอภา วัชระคุปต์, ปรีชา บุญจูง, จันทนา บุณยะรัตน์, มาลีรักษ์ อัตต์สินทอง. สารต้านอนุมูลอิสระ. กรุงเทพฯ: 2549. พี.เอส.พริ้นท์;.พิมพ์ครั้งที่ 1.
- อนงนาฎ ไพนุพงศ์,2017, อนุมูลอิสระและสารต้านอนุมูลอิสระกับสุขภาพ, PKRU Scitech journal, Vol1(2), 20-27.