
Information on Anti-Tyrosinase Enzyme Inhibition Testing Services
VISBIO offers services for testing and analyzing the inhibitory effects of Anti-Tyrosinase enzymes on various products. This testing is suitable for products in the health and beauty industry that focus on properties like skin whitening, reducing dark spots, and more. Tyrosinase is an enzyme responsible for catalyzing the production of melanin, which is related to the occurrence of skin darkening and the formation of dark or brown pigment granules in the skin or hair.
The process of melanin synthesis occurs through the action of Tyrosinase, the enzyme with the most significant role in melanin granule synthesis. There are several types of substances that can inhibit the activity of Tyrosinase, both natural and synthetic ones. Therefore, testing the inhibition of Tyrosinase activity with products is suitable for those that excel in skin whitening, reducing freckles, dark spots caused by acne, and more.
Tyrosinase
Tyrosinase is an enzyme found in various living organisms, including bacteria, fungi, plants, animals, and humans. It accelerates the reaction in the production of the pigment melanin, which is related to the darkening of skin color in fruits and vegetables. In some insects, this enzyme is essential for growth, particularly during metamorphosis from larvae to adults. In humans, the enzyme tyrosinase is synthesized from ribosomes, found in the rough endoplasmic reticulum, and transported by the Golgi apparatus to melanosomes. Its main function is to synthesize melanin, contributing to the formation of black or brown pigment granules in the skin and hair.
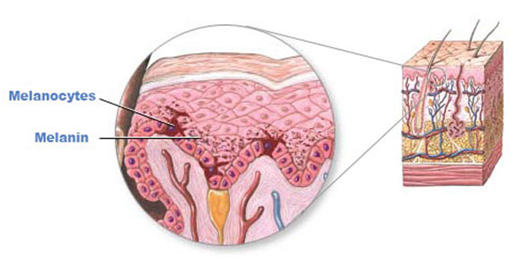
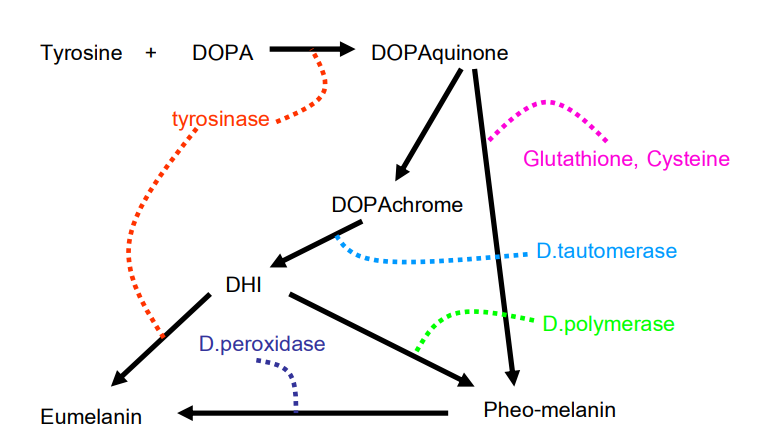
Melanin biosynthesis occurs through the enzyme tyrosinase, which plays a crucial role in synthesizing melanin granules. It converts the precursor tyrosine into 3,4-dihydroxyphenylalanine (DOPA), which is further transformed into Dopaquinone. Dopaquinone then reacts with the enzyme glutathione or cysteine, resulting in the conversion into Cysteinyl DOPA and forming yellow/red pheomelanin.
In the absence of cysteine, Dopaquinone is converted into DOPA achrome, which subsequently changes into DHICA. The enzyme Tyrosinase-related protein 1 (TYRP1) further accelerates the reaction of DHICA, leading to the formation of black eumelanin. The whole process is illustrated in figure 1.
Group of substances that inhibit the function of tyrosinase enzymes.
These substances can be classified into two main groups based on their sources:
- Natural substances, including Kojic Acid, Arbutin, and polyphenols such as flavonoids, gallic acid, ellagic acid, resveratol, and oxyresveratrol. These substances are commonly found in vegetables, fruits, and flowers with colors, for example, berries, green tea, pomegranate, apples, grapes, strawberries, and mulberries. Additionally, there are substances like benzaldehydes and benzoate derivatives.
- Synthetic substances, including N-(phenylalkyl) cinnamides, sildenafil, and oxadiazole.
Testing methods for inhibitory effects on tyrosinase enzyme function
There are two testing methods used for determining the inhibitory effects on tyrosinase enzyme function:
- In vitro test, which includes the mushroom tyrosinase inhibitory assay and modified dopachrome. This test calculates the half maximal inhibitory concentration (IC50) and cellular tyrosinase inhibitory assay.
- In vivo test, which involves testing depigmenting activity in living organisms such as mouse models, zebrafish models, and human skin.
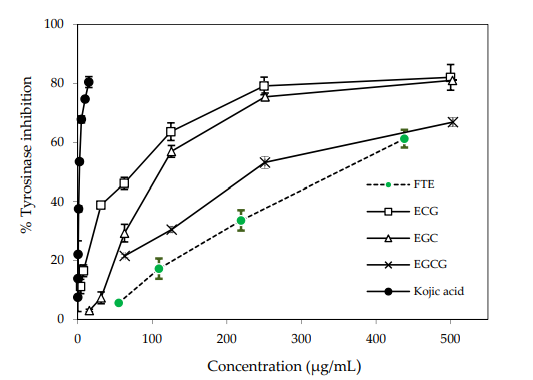
Therefore, testing the inhibitory effects of Tyrosinase with significant substances or extracts is suitable for the cosmetic industry, particularly in products that focus on skin whitening, reducing freckles, spots caused by acne, and achieving clear and radiant skin. An example of the test results on the tyrosinase inhibition of Camellia sinensis leaf extract (FTE) and various catechins (epigallocatechin gallate – EGCG, epigallocatechin – EGC, epicatechin gallate – ECG) yielded an IC50 value of 2.28 µg/ml for Kojic acid (Surached, 2017).
Literature:
- Surached Thitimuta, 2017, Camellia sinensis L. Extract and Its Potential Beneficial Effects in Antioxidant, Anti-Inflammatory, Anti-Hepatotoxic, and Anti-Tyrosinase Activities, Molecules 2017, 22, 401.
- Khwunjit Itsarasook, 2015, Free Radical Scavenging and Tyrosinase Inhibitory Activity of Longkong Leaves Extract, SDU Res. J.8(3).
- Sawitri Tasutin, 2006, Preliminary screening of medicinal plants for anti-tyrosinase activity, Special Problems Bachelor of Science. อำไพ พฤติวรพงศ์กุล, พิมพร ลีลาพรพิสิฐ และ ทักษอร รัตนยุวัน, 2557, การพัฒนาตัวพาอนุภาคนาโนไขมันที่บรรจุสารสกัดดอกดาวเรืองเพื่อยับยั้งฤทธิ์เอนไซม์ไทโรซิเนสม, วิทยานิพนธ์ มหาวิทยาลัยเชียงใหม่.

